隔膜泵是用于抗击2019新冠肺炎医疗设备中的关键部件

人的心脏是大自然的奇迹。它每天连续不断地跳动多达 100000 次,对于一个健康的人而言,这是理所当然。然而,对于急需新心脏的心脏病患者,情况则完全不同。他们的存活机会很大程度上取决于能否找到合适的捐献者心脏。但是等待人的名单很长。心室辅助装置(VAD)可以帮助病人度过临时过渡期。 Berlin Heart 的 EXCOR® 儿科心室辅助装置是全世界唯一被批准用于儿童和婴儿的心室辅助装置。KNF为其固定驱动器提供高度专业的隔膜气泵。
从初创公司到欧洲心室辅助设备行业的领导者
国际知名的医疗器械制造商柏林心脏有限公司(Berlin Heart GmbH)总部设在柏林。它成立于80年代末,在柏林以德国心脏中心的一个分公司的形式出现。公司内云集了临床医生和开发人员,而且在 Fehling Medical 公司的支持下,最终于 1996 年以目前的形式成立了 Berlin Heart。同时,该公司是欧洲心室辅助设备的先驱和市场领导者,拥有200多名员工。
机械式血泵模拟心力衰竭患者的心脏功能
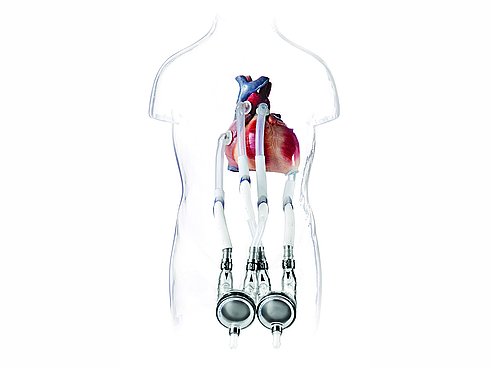
该公司的核心产品是EXCOR®血泵,一种双腔泵,为心力衰竭患者实现心脏功能。该泵被放置在患者身上,并通过小管连接到心脏和血管。驱动单元通过压缩机产生压力和真空,为三层隔膜提供动力,隔膜将气室和血室分开,膜片以脉动的方式运动,继而实现血液流动。
在1990年代, Berlin Heart 通过开发 EXCOR®Pediatric(儿科心脏泵)取得了全球性突破,这是首款为儿童治疗而开发的产品。当时,该公司正在寻找一个可靠的合作伙伴,以便为相关的 Ikus 固定式驱动装置开发合适的压缩机。1993 年,Berlin Heart 最终发现了隔膜泵制造商 KNF。
经过验证的质量和可靠性,使项目得以快速实施
由于治疗可能需要数月甚至数年的时间, EXCOR®的所有组件均设计为长期使用。因此,为了开发用于驱动单元的 KNF 隔膜气泵,这家医疗技术制造商提出了明确的规定。 为了改善治疗过程中患者的生活质量,除了性能和使用寿命,安静的运行也很重要。考虑了所有方面后,KNF的隔膜泵技术可以完全满足 Berlin Heart 开发人员的所有要求。合作还得益于可靠的“KNF模块化系统”。
KNF 模块化系统使用经过检验的泵组件,这些组件已根据 Berlin Heart 的个别项目要求进行了调整。“尽管我们提出了特殊要求,但无需完全重新开发隔膜泵,” Berlin Heart 的维护团队负责人 Daniel Hartmann 兴奋地说。“相反,我们能够使用 KNF 模块化系统中经过验证和可靠测试的组件。在我们想在短时间内开发出可靠的驱动系统之际,这大有裨益。”
借助于模块化系统,Berlin Heart 和 KNF 开发团队共同优化了参数,直到让“N 023.1.2”泵的系列型号完美满足客户的特定要求。在其他改进中,采用了特别调整的中间板和特殊的硅材料连接管路。通过精心协调所有组件,Berlin Heart 的 EXCOR®儿科产品在美国市场上也获得了完全的上市许可(Premarket Approval,PMA)

挽救生命 —— 企业成功的动力
经过两年的深入开发工作,应用结果也令人振奋:自 1994 年以来,唯一获准用于儿童、幼儿和婴儿的心室辅助装置挽救了 2500 多条生命。“当市场传来关于成功应用的反馈,尤其是关于孩子的消息时,您总会获得新的力量和快乐。这正是我们在 Berlin Heart 的主要推动力,” Hartmann 坦言道。多年来,该公司已经能够开拓越来越广阔的市场。子公司 Berlin Heart Inc.于 2005 年在美国成立,EXCOR® Pediatric 也在那里获得了完全的 FDA 市场准入(售前许可,PMA)。2015 年,首批日本患者成功使用系统进行了治疗。如今,亚洲市场已成为 EXCOR® Pediatric 的最大用户群之一。目前,人造心脏已成功应用于所有大陆。
新市场的开发需要不断的发展
随着新市场的开发,对心脏泵的要求也在发生变化。例如,必须根据不同的国家实施具有适当电压的驱动系统。在这方面,Berlin Heart 也可以依靠 KNF 的专业知识。根据各种新要求,KNF 在不断开发解决方案。“现在,我们有五种不同的电压类型;因此我们事实上可以在世界上的几乎任何地方使用我们的系统,”Hartmann 表示。“在整个项目过程中,KNF 始终关注我们的问题和疑虑,并积极采取措施加以解决。此外,我们一直感到我们的应用受到了真正的推崇。迄今为止,合作非常愉快!”